If you’ve been searching for the next big thing in weight loss, retatutride might just be it. Developed by pharmaceutical giant Eli Lilly, this experimental weight loss drug is making waves as it moves through phase 3 clinical trials in March 2025. With obesity rates soaring—over 40% of U.S. adults are obese, according to the CDC—the demand for effective solutions has never been higher. Current options like semaglutide (Ozempic/Wegovy) and tirzepatide (Mounjaro) have set a high bar, but retatutride’s early results suggest it could very well outshine them all.
What makes retatutride stand out is that it’s a triple agonist weight loss drug, targeting three hormones at once—GLP-1, GIP, and GCGR. This innovative approach promises not just to curb appetite but to rev up metabolism in ways we haven’t seen before from this class of drugs. As researchers dig into the results of retatutride’s phase 2 trials, the buzz is growing: Could this be the game-changer we’ve been waiting for in obesity treatment?
Before we dive into why retatrutide may be better than ozempic, let’s step back and look at the broader class of drugs it belongs to—GLP-1 agonists.
The GLP-1 Drug Class – A Revolution in Weight Loss
To understand retatutride, you need to know the family it comes from: the GLP-1 agonist class of drugs. These medications started as diabetes treatments, but their weight loss potential quickly stole the spotlight. They work by mimicking a hormone called glucagon-like peptide-1, which your body naturally produces after eating, to regulate appetite and blood sugar.
Drugs like liraglutide (Saxenda) kicked things off, followed by semaglutide (Wegovy/Ozempic), which became a household name after showing 10-15% body weight reductions in trials. Then came tirzepatide (Mounjaro), a dual agonist adding GIP to the mix, pushing weight loss even further—up to 20% body weight reduction over the course of 72 weeks in some cases.
This GLP-1 drug class works by tapping into your body’s natural systems. GLP-1 agonists tell your brain you’re full, slow down digestion so you stay satisfied longer, and help your pancreas manage insulin. Some newer drugs in this class also target the gastric inhibitory polypeptide receptor, which further suppresses appetite while also modestly increasing energy expenditure.
These drugs have transformed obesity care, earning FDA approval and millions of users. NBC News called them a “new era” in weight management, and they’re not wrong—sales of semaglutide alone have soared to over ten billion a year despite increasing competition from newer drugs in this class.
And that leads us to retatutride. Let’s break down how this new obesity treatment works and why it’s generating so much excitement.
How Retatutride Works – Triple Power Unleashed
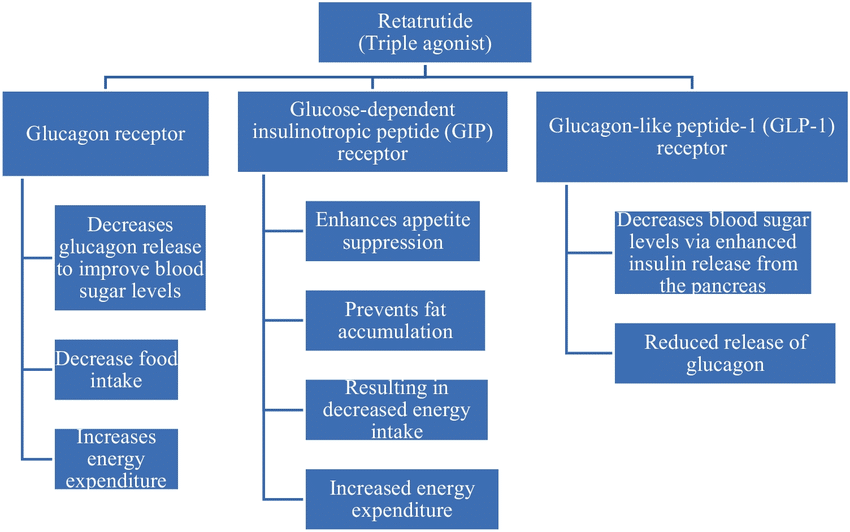
So, what makes retatutride different from the pack? It’s all about its triple-threat approach. As a triple agonist weight loss drug, retatutride targets three hormones—GLP-1, GIP, and GCGR—giving it a unique edge over single- or dual-agonist drugs in the GLP-1 agonist family. Delivered via a weekly subcutaneous injection (just under the skin), it’s designed to tackle the problem of weight loss from multiple angles at once.
Here’s how it breaks down: GLP-1 curbs your appetite by signaling fullness to your brain and slows digestion, so you feel satisfied longer—familiar territory for drugs like semaglutide. GIP, seen in tirzepatide, amps up fat metabolism and insulin sensitivity. Then comes GCGR, the wildcard retatutride adds to the mix, boosting energy expenditure by revving up your body’s calorie-burning engine. ResearchGate offers a handy diagram of this mechanism, showing how these hormones team up to shrink waistlines.
This triple power sets retatutride apart from its Eli Lilly weight loss predecessor, tirzepatide, and competitors like Wegovy. While dual agonists stop at appetite and fat, retatutride’s GCGR action could mean more fat burned even at rest—a potential game-changer. But how does this translate to real results? Let’s look at what the retatutride trials have uncovered so far.
Research Insights – What We Know So Far
The hype around retatutride isn’t just talk—it’s backed by solid data from phase 2 retatutride trials. The big reveal came in a phase 2 study published in the New England Journal of Medicine in June 2023. This multicenter, randomized, double-blind trial tested retatutride on 338 adults with obesity or overweight (BMI ≥ 27) and at least one related health issue, excluding type 2 diabetes. Participants got weekly doses ranging from 1 mg to 12 mg or a placebo for 48 weeks. The results? Staggering.
At the highest dose (12 mg), people lost an average of 24.2% of their body weight by 48 weeks—that’s nearly a quarter of their starting weight. Even at 24 weeks, the 12 mg group dropped 17.5%, blowing past placebo’s 1.6%. NBC News called it a potential “king” of weight loss drugs, and the numbers back that up. Side effects were mostly gastrointestinal—nausea, vomiting, diarrhea—but mild to moderate, fading over time without major dropouts. No serious red flags emerged, a good sign for safety.
Now, retatutride is in phase 3 trials, set to wrap up by late 2025, according to Clinical Trials Arena. These studies, like TRIUMPH-3 and TRIUMPH-4, are testing it in larger groups, including those with cardiovascular disease and osteoarthritis. They’ll answer big questions: Does it hold up long-term? Are there rare retatutride side effects we’ve missed? How does it impact heart health or joint pain? The FDA’s drug approval process will hinge on these outcomes. For more on clinical trials, see our Unwinder explainer on how drugs get approved (insert-internal-link-here). Next, let’s see how retatutride measures up to its rivals.
Retatutride vs. Ozempic and Tirzepatide
How does retatrutide compare to the heavy hitters in the weight loss drug arena? Let’s put it head-to-head with semaglutide (Ozempic/Wegovy) and tirzepatide (Mounjaro), two approved stars in the GLP-1 agonist class. The numbers tell a compelling story. In its phase 2 trials, retatutride hit 17.5% weight loss at 24 weeks with its 12 mg dose (NEJM, 2023). Compare that to semaglutide’s 10.2% at the same point (NEJM, 2021) or tirzepatide’s 10.5% (NEJM, 2022), and retatutride looks like it’s pulling ahead early.
Retatrutide’s mechanism of action matters too. Semaglutide, a single GLP-1 agonist, focuses on appetite control and digestion slowdown. Tirzepatide, an Eli Lilly weight loss success, adds GIP for a dual-agonist punch, boosting fat metabolism. Retatutride’s triple agonist weight loss approach—GLP-1, GIP, and GCGR—throws in glucagon to crank up energy expenditure in addition to further suppressing appetite.
That extra kick might explain its edge, potentially burning more calories even when you’re Netflix-binging. Retatrutide also shows at least two other benefits of note. First, people are less susceptible to rebound weight gain after discontinuing it. Rebound weight gain does happen, but it’s delayed by a few months and occurs more slowly. This is most likely because retatrutide increases physical activity, helping patients maintain muscle mass and thereby keeping their basal metabolic rate high.
Second, retatrutide shows promise in slowing the progression of obesity-related cancers. It can’t cure cancer, but it could end up becoming a standard adjunct to chemotherapy in certain types of cancer.
ScienceDirect notes this triple combo could redefine obesity treatment, but it’s not all rosy. Side effects are a trade-off. All three drugs bring nausea and digestive woes—retatrutide side effects mirror its rivals, though its phase 2 data suggests they’re manageable. Does the bigger weight loss justify the hype? Things look promising, but we’re still waiting on phase 3 trials to fully understand the long-term effects.
Surprisingly, that doesn’t mean you can’t get a hold of retatrutide now.
How To Obtain Retatrutide Now
Although retatrutide is not yet FDA-approved, there is an exception to FDA approval whereby non-approved chemicals can be sold online purely for research purposes. A number of websites, collectively known as research chemical sites or peptide sellers, abuse this exception to sell unapproved drugs like retatrutide, as well as selling approved prescription drugs like viagra without a prescription.
The Unwinder is not in a position to recommend any of these sites, nor verify the authenticity or purity of the retatrutide they have on offer. You can find them easily enough by googling “buy retatrutide online,” “retatrutide peptide,” or “retatrutide research chem.”
Be aware that retatrutide is sold in injectable form. To use it, you’ll need to buy a vial of crystalized retatrutide, a vial of bacteriostatic water, some insulin needles and sterile alcohol wipes. You’ll have to inject the bacteriostatic water into the retatrutide and let them mix, then store the mixture in your fridge. You also need to make sure sure it doesn’t freeze, which will destroy or degrade the drug or possibly crack the vial. Actually taking the drug will involve injecting it once a week subcutaneously, into your abdominal fat.
These peptide sites are, needless to say, a bit sketchy. While all sorts of people use them, their core customers seem to be biohackers and steroid abusers. Their products are often made with precursor chemicals sourced from China (The Unwinder occasionally gets spam emails from the sellers of these precursors), and while research chemicals usually contain what they purport to contain, they’re not always pure. Buyer beware.
The Road Ahead For Retatrutide
So, when might retatutride hit the market as a new obesity treatment? As of March 2025, it’s deep into phase 3 trials, with completion slated for late 2025 (Clinical Trials Arena). Assuming solid results, Eli Lilly could file with the FDA by early 2026. The FDA’s standard review takes 10 months, but priority status—common for obesity breakthroughs—could shrink that to 6 months, landing retatutride FDA approval in mid-2026 or early 2027. On the other hand, dysfunction at the FDA or a change in approval policies could always delay approval into 2027.
The odds of retatrutide’s eventual approval are likely around 60-70%. That’s higher than average thanks to its proven GLP-1 agonist class roots and predecessors like tirzepatide demonstrating the safety and efficacy of this overall class of drugs.
Phase 3 will seal the deal—or break it. These trials are digging into long-term safety, efficacy in diverse groups (think heart disease or arthritis patients), and whether that 24.2% weight loss holds up. Rare retatutride side effects or unexpected flops could derail it, but its class gives it a leg up. PMC highlights its potential as a “triumph over obesity”, though we’re still waiting for the full picture.
So is retatrutide better than Ozempic? Probably, but we need another year to be sure. Retatutride could redefine weight loss drug options if it clears phase 3 and earns approval. Stay cautious about peptide shortcuts and keep an eye on the outcomes of retatutride’s phase 3 trials come late 2025 or early 2026.
Further Reading on Weight Loss Drugs & Supplements
Does Adderall Make You Lose Weight?
Can Cinnamon Really Help With Weight Loss?
Lithium Orotate Weight Loss Benefits
The Best Glucomannan Pills For Weight Loss